UNE researchers publish novel approach for assessing neonatal mouse heart function
The study may help clinicians identify congenital heart defects in, and develop treatments for, babies even before birth

Researchers in the Tucker Laboratory at the University of New England have published a novel exploration into the assessment of neonatal, or newborn, mouse heart function using a noninvasive approach to monitoring the electrocardiography (ECG) of baby mice (pups).
The publication in the Journal of Visualized Experiments (JoVE), “Noninvasive Electrocardiography in the Perinatal Mouse,” was written in collaboration with current and past UNE College of Osteopathic Medicine (COM) students, Kerry L. Tucker, Ph.D., associate professor in the Department of Biomedical Sciences, and Adrian Moran, M.D., a pediatric cardiologist at Maine Medical Center.
The paper presents a first-of-its-kind, noninvasive process for measuring the electrical activity of newborn mice with the goal of detecting congenital heart defects (CHD) in the tiny mammals as early as the first day after birth.
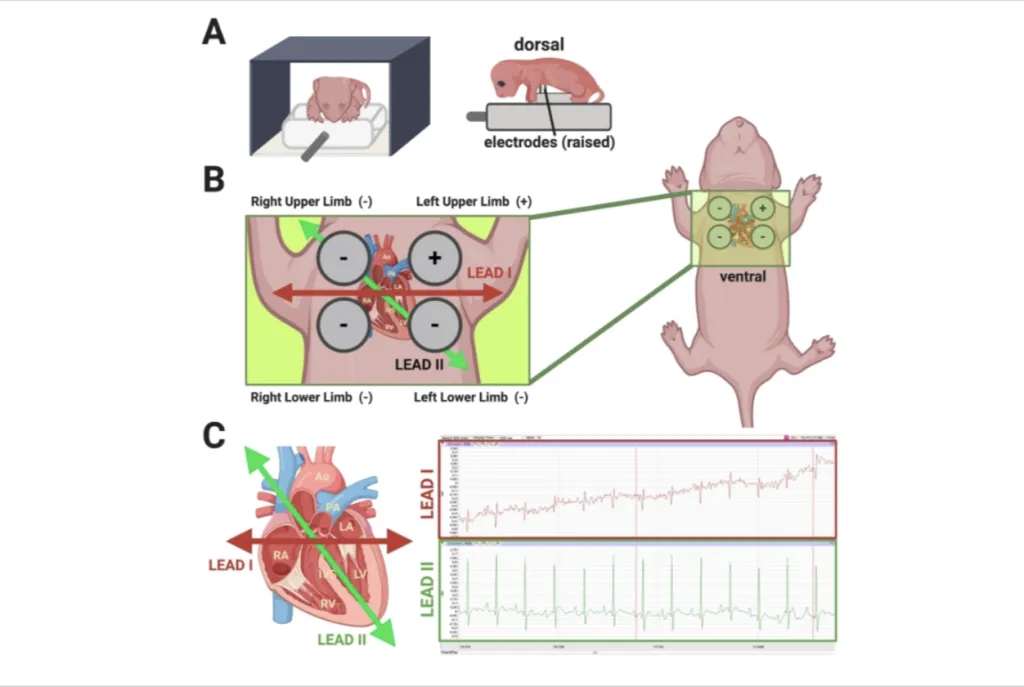
The process utilizes a device developed in partnership with Dover, New Hampshire-based physiology research and teaching firm iWorx. It employs ECG technology located inside a plastic platform. Four electrodes rise above the platform to measure the neonate’s heart activity while cushioned bumpers on either side of the mouse keep it steady. The entire unit, which is heated for the mouse’s comfort, is shielded from the light to protect the pup’s eyes while the ECG is performed.
The gentle technique has not been used before on neonatal mice, which are too small to undergo a typical ECG. Other methods exist for tracking heart activity in mice, but they often require anesthesia or use of a needle to measure electrical signals, which can be traumatic for newborn mice, regardless of CHD status.
“The newborn mice we study have a particular form of CHD that causes the pup to die within the first day of life,” the researchers explained. “This new technique allows us to monitor the electrical signals of the heart in order to understand why and how CHD changes the heartbeat in a way that is not compatible with life.”
The protocol, the collaboration of researchers write, was designed to directly address a need for a standardized and repeatable method for obtaining ECGs in newborn mice. Tucker, the UNE professor, credits the protocol as being the brainchild of the study’s main research scientist Lindsey Fitzsimons, M.S., RCEP/CES, who is a Ph.D. candidate at the University of Maine Graduate School of Biomedical Science and Engineering who is completing her doctoral thesis at the Tucker Lab.
Fitzsimons said she hopes the method and subsequent research manuscript will bridge a gap in the scientific and medical literature about the functional measurement of the heart in studies using mice.
“The reason we started trying to develop this technology was because I realized that of all the extensive research looking at congenital heart defects in mice, very few studies include measurements from the physiology, or function, of the CHD heart,” she said.
Specifically, Fitzsimons added, she hopes to see an increase in the number of scientific studies documenting and characterizing the functional measurement of the newborn mouse heart so that this research may be applied further towards studies performed in newborn humans.
“The more parallels we can draw with rodent and human cardiac function, the greater the reproducibility and translatability of both basic science and clinical biomedical research,” she remarked.
The novel research builds on existing studies out of the Tucker Lab, an interprofessional laboratory whose research focuses on better understanding the cellular processes that lead to the development of CHD through the convergence of embryology, molecular biology, clinical cardiology, and neuroscience in collaboration with lab members from disciplines across UNE COM and the University’s Colleges of Arts and Sciences and at Maine Medical Center.
Congenital heart defects are currently the most common birth defect, occurring in approximately 1 in 100 live births in the United States each year. About 25% of babies born with CHD have a defect considered to be life-threatening, according to the U.S. Center for Disease Control and Prevention, and — while there are surgical and medical management options available to patients — clinicians and researchers agree that a full understanding of the molecular and cellular processes leading to the disease will refine future treatment options.
Enter the Tucker Lab at UNE.
The lab’s research has focused mainly on the primary cilia, which are physical, finger-like structures located on the surface of many cell types that help to control the key sensory and signaling patterns essential to developing tissue, among other functions. Specifically, Tucker’s lab is working to understand how damage to these tiny structures causes cells in the developing heart to behave erratically, leading to cellular disorganization, changes in gene expression, and improper alignment of both the heart itself as well as the associated blood vessels, directly resulting in CHD.
Fitzsimons said she hopes the research — and the patent-pending iWorx system developed in the process — will help expand the breadth of scientific inquisition into CHD with the goal of better understanding the risk factors, drug targets, and various treatment options for this disease, which may be tested and applied across species.
“In spite of known risk factors, such as the environment, age, race, genetics, and family medical history, human congenital heart defects are not often discovered until late in pregnancy and/or even after a baby is born,” Fitzsimons said. “We hope this research will allow clinicians to identify CHD earlier on with the goal of establishing the best treatment for babies even before birth.”